Presented at:


Bare® Clinical Trials 2016, Brief Parent Report (pdf_report | pdf_poster)
Evelyn Ramirez-Coombs1
1Coombs Research Analytics
I. Introduction
Acid reflux, also known as gastroesophageal reflux (GER), describes the abnormal ascension of hydrochloric acid from the stomach to the esophagus. GER causes inflammation of the esophageal lining, pyrosis, extreme pain when eating and inconsolable crying in infants. Research indicates 2/3 of 4-month olds have symptoms of gastroesophageal reflux disease (GERD) which can lead to acidic taste at the back of the mouth, chest pain, breathing difficulties, speech impediments, tooth decay, esophageal stricture, and Barrett syndrome. However, despite overwhelming evidence suggesting a robust relationship between GERD and negative health outcomes, there is still a major void in the literature, and the technological market to address the feeding needs of children with acid reflux. While different groups have developed variations of disposable liners and vented technology to relieve feeding discomfort for infants, clinical observations indicated the persistence of air infiltration into the bottle, leading to gas build-up, pain, and worsening acid reflux upon ingestion. We believe providing air-free feeding along with pediatrician recommended upright feeding positions, as well as avoiding overfeeding, will be imperative in treating acid reflux in infants, and revolutionizing the medical and technological approach to pediatric feeding.
- Research Question
Can new air-free feeding technology such as Bare®Air-Free help attenuate GER symptoms in infants with GERD symptomology ages 0-18 months? - Hypothesis
We hypothesize that Bare® Air-Free will reduce GERD symptoms in infants exhibiting GER symptomology ages 0-18 months - What is Bare® Air-Free?
This novel feeding system provides pioneering technology offering three major advancements that exponentially reduce reflux symptoms in babies.
Bare® Air-Free enables babies to:

- Feed in the upright position, reducing regurgitation of stomach acids into the mouth
- Ensures Air-free feeding
- Allows the baby to create suction in order to feed and control the pace of milk outflow, thus, discouraging over-feeding behaviors
Preliminary studies analyzing parent feedback of Bare® Air-Free found that 60% of parents reported a reduction of air in the infant’s milk, and qualitative findings indicated improvement in acid reflux symptoms. In light of this overwhelming feedback from parents, we felt it was our responsibility to investigate this phenomenon, by conducting a clinical study to determine if there is, in fact, a link between the use of Bare® Air-Free and the reduction of acid reflux in babies through the design of an experiment. We also aimed to quantify this effect size through the use of an empirically valid measure of GER in babies.
II. Methods
- Participants
Participants were recruited through an online-ad posted on Bittylab.com and other popular social media websites. A total of (N = 422) caregivers responded to the ad and completed the pre-test measure. After screening every entry, a total of (n = 68) participants who met clinical criteria for GER via the I-GERQ, were placed in the GER group. All age and gender-matched controls who did not meet the criteria for GER were placed in the Control group (n = 54), resulting in a total of (N = 122) enrolled participants. Exclusion criteria included any participants with missing data, mother’s use of drugs, alcohol or nicotine while pregnant, and any medical or neurological disease, as well as developmental disorders. Inclusion criteria included having a baby, babies with typical feeding behaviors, as well as babies with symptoms of acid reflux. - Measures
The Infant Gastroesophageal Reflux Questionnaire-Revised (I-GERQ-R) is a 14-item caregiver report questionnaire using a Likert scale to quantify the degree of feeding discomfort the infant or child is currently experiencing. The I-GER-Q is an empirically validated measure of GERD in babies and has been regularly used for evaluative purposes in clinical trials. The clinical cut off score for GERD on the I-GERQ-R is 16. The I-GER-Q has demonstrated strong internal consistency reliability (0.87), and test-retest reliability (0.85), as well as adequate construct validity. Demographic information such as age, gender, a preferred method of feeding, etc. was also collected. - Procedures
Participants were recruited online, via ads on Bittylab.com and various social media outlets. Prior to participating in the clinical study, all participants were required to provide written consent and a release of liability. All interested caregivers completed various demographic questions and completed the I-GERQ-R (pre-test). All entries were screened, and babies meeting clinical criteria for GERD (I-GERQ-R raw score ≥ 16) were placed in the GERD group (n = 68). All other age and gender-matched babies who did not pass the clinical cut-off score were placed in the Control group (n = 54). Selected participants were then asked to watch an instructional video on Bittylab.com which provided step-by-step instructions on how to properly assemble and utilize the Bare® Air-Free feeding system (e.g. always feed the baby in an upright position; never push forward the plug, etc.). At the end of the video, caregivers were provided with a code that they had to email to the research coordinator in order to proceed with the study. Upon receipt of the training video code, participants were then shipped a Bare® Air-Free feeding system, free of charge. Caregivers were instructed to utilize only Bare® Air-Free for the following 2-weeks, as well as referring to the training video whenever need, to secure correct assembly and use of the device. After 2-weeks, all participates received the follow-up questionnaire (post-test) via email. The follow-up questionnaire consisted of additional demographic questions (e.g. did you only use Bare® Air-Free when feeding?), and the same I-GERQ-R completed at baseline.
III. Results
- Descriptives
A total of (N = 122) participants were recruited for the study. At baseline, the overall sample had 56 female babies and 66 male babies, with ages ranging from 0-18 months, and a median age of 0-3 months (75%). Approximately 99% of caregivers reported being mothers. The GERD group had a total of (n = 68) participants, while the control group had a total of (n = 54).
At the end of the 2-week clinical trial, a total of (N = 69) participants responded to the follow-up questionnaire, representing a 57% retention rate. Furthermore, attrition rate was equal between groups, where the GERD group had a 57% retention rate, and the CTL group had a 56% retention rate. - Analyses
Baseline (pre-test) and follow-up (post-test) data for both the GERD and CTL group were analyzed using IBM SPSSS, a statistical package. Group means were compared using a repeated measures ANOVA. - Major Findings
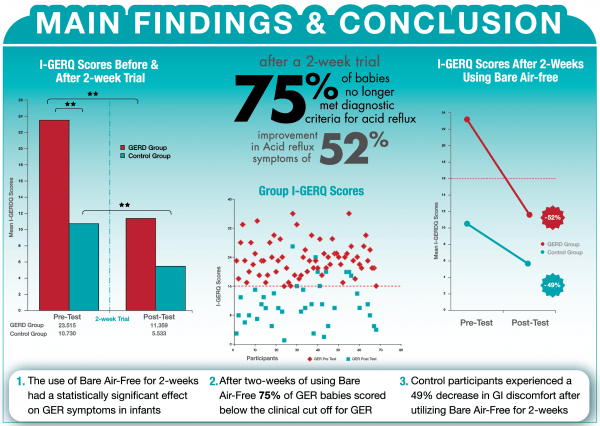
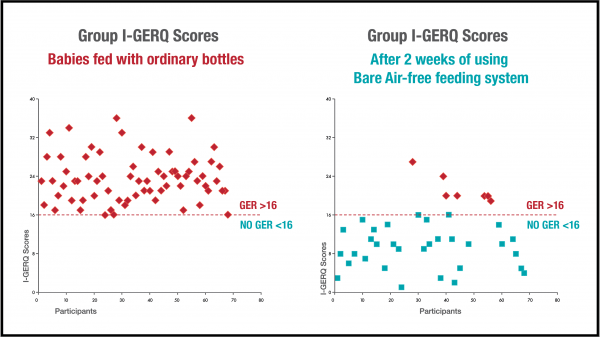
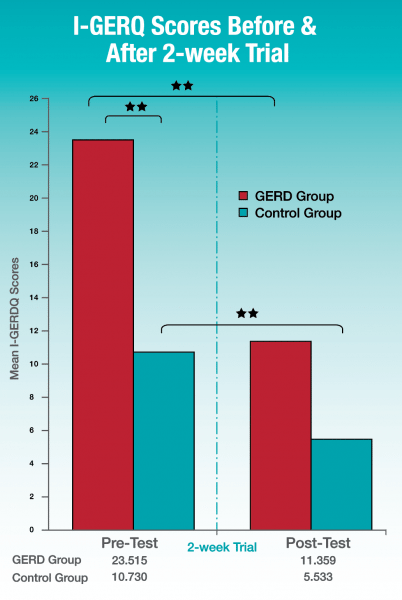
A repeated measures ANOVA revealed a statistically significant difference between pre- and post- measures on the I-GERQ-R for both the GERD and CTL group (p < .01), where mean GER scores in the GERD group declined from (M = 23.51, SD = 4.82 ) to (M = 11.36, SD = 6.21), reflecting a 52% reduction in GER symptoms after 2-weeks of using Bare® Air-Free. Furthermore, 75% of babies previously of babies in the GERD, no longer met criteria for GERD. In addition, mean GER scores in the CTL group also declined from (M = 10.73, SD = 3.38) to (M = 5.53, SD = 5.21), reflecting a 49% reduction in GER symptoms.
IV. Discussion
- Major findings
In summary, the use of Bare® Air-Free for 2-weeks had a statistically significant effect on GER symptoms in infants and babies. After two weeks of using Bare® Air-Free, babies with GERD experienced a 52% improvement in GER symptoms. Furthermore, up to 75% of GERD babies scored well below the clinical cut off for GERD after only 2-weeks. What was even more surprising was the unexpected attenuation of GI discomfort in CTL babies as well, suggesting Bare® Air-Free has benefits for babies with various profiles and severity of feeding discomfort.
- Clinical Implications
These findings are imperative to the advancement of pediatric feeding technology, and patient care. These results suggest the combined application of air-free feeding, upright positioning, as well as the baby’s control of flow and pace work as a system to reduce instances of acid reflux. - Limitations
As part of the preliminary stages of a larger study, this experiment was subject to various limitations, such as the moderate sample size, which is likely due to the short time in which the study was conducted (about one month). In addition, there was no way of confirming that all caregivers used only Bare® Air-Free for two-weeks, as well as properly using the feeding system. In addition, we were unable to acquire at least 70% retention rate, however, this is likely due to the short time-frame of the study and outreach during the follow-up phase, which lasted approximately a week and a half. - Future Studies
Our next step in this line of research is to conduct a larger scale study to investigate how this technology can further benefit infants and babies with various feeding difficulties.
V. References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.)
- Benjasuwantep, B., Chaithirayanon, S., & Eiamudomkan, M. (2013). Feeding Problems in Healthy Young Children: Prevalence, Related Factors and Feeding Practices. Pediatric Reports, 5(2), 38–42. https://doi.org/10.4081/pr.2013.e10
- Czinn, S., J., & Blanchard, S. (2013). Gastroesophageal reflux disease in neonates and infants: when and how to treat. Peadiatric Drugs, 15(1)19-27.
- Manikam, R. & Perman, J., A. (2000). Pediatric Feeding Disorders. Journal of Clinical Gastroenterology, 30(1), 34-46.
- Vandenplas, Y., Rudolph, C., D., Di Lorenzo, C., (2009). Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Journal of Pediatric Gastroenterology and Nutrition, 1(49), 498-547.
Poster

Opinion
BRUCE OGDEN MD FAAP
Neonatology, Pediatrics & Pediatric Hospitalist
Level III Neonatal & Perinatal Care, Physician & Owner of Bruce Ogden MD
While the medical community does not know the exact cause of GER and GERD in infants, there are many hypotheses that explain possible causes for reflux. One of them is that air-swallowing might be responsible for a small part of this. If this was true, eliminating air from the feedings –and therefore from the stomach—one would have expected a small effect. However, after seeing the monumental positive results after eliminating air from the feedings, the air has to play a MAJOR role in reflux or it may be the single, largest factor in causing GERD. This makes this pilot study the first of its kind and could help the medical community discover this new side to reflux. As researchers, we have the responsibility and obligation to learn more about this device and its positive outcomes in the full-term and premature infant population. Furthermore, one can hypothesize that what allows reflux to happen is when the pressure within the stomach builds up until it’s greater than the pressure of the LES muscle trying to keep the esophagus closed. So when infants swallow air, especially while feeding, it distends the stomach, and pressure in the stomach rises until it overcomes the muscle sphincter and reflux happens.
